Introduction: The combination of hypomethylating agents with venetoclax (HMA+VEN) has demonstrated preferential activity in patients (pts) with isocitrate dehydrogenase (IDH) 1/2 mutated acute myeloid leukemia (AML). With the increasing availability of single and combination therapies including oral small-molecule inhibitors of IDH1 and IDH2, respectively, additional detail regarding depth of remission and duration of response (DOR) with the various combinations of active agents in larger datasets are desired.
Methods: We reviewed pts with newly diagnosed (ND) and relapsed/refractory (R/R) IDH1/IDH2 mutated AML treated with HMA+VEN at our institution between 2014 and present. A subset of these pts received IDH inhibitor (IDHi)-based regimens at alternate timepoints; these data were used for within-patient comparisons. Responses were defined by 2017 ELN criteria with the additional incorporation of measurable residual disease (MRD) assessment by flow cytometry (MRD-FC, sensitivity 10-3-10-4). Mutant IDH (mIDH) variant allele frequency (VAF) from amplicon-based next-generation sequencing (NGS) of bone marrow samples performed pre-treatment, at best response, and other available timepoints were analyzed for MRD by NGS (MRD-NGS, limit of detection 1-2%). OS was defined as the time from start of HMA + VEN to death or last follow up.
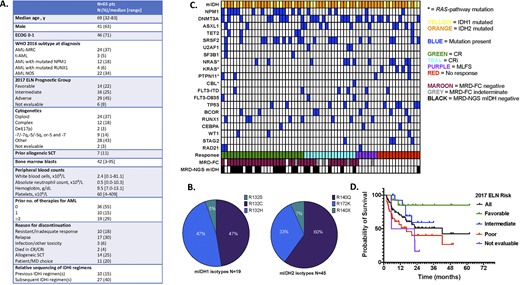
Results: Sixty-five pts (36 ND, 29 R/R; 45 mIDH2, 19 mIDH1, 1 both isotypes) received HMA+VEN; pre-treatment characteristics are shown in Figures A and B. Median age was 69 years; 45% percent had ELN adverse risk disease. Most frequent co-mutations (Figure C) were SRSF2 (42%), DNMT3A (38%), and NPM1 (28%). After a median of 1 cycle (range 1-5), ORR for HMA+VEN received as any line of therapy was 79% (CR 42%, CRi 26%, MLFS 11%, PR 0%). Ninety percent (36/40) of CR+CRi responses with evaluable MRD-FC were MRD negative. CR+CRi rates were 86% in ND pts and 100% in ND pts with mutated NPM1. Presence of a RAS-pathway and/or TP53 mutation was associated with a trend to lower likelihood of CR/CRi in R/R disease (35.7% vs. 53.3%, p=0.46). Fifty-two percent (23/44) of pts with available longitudinal mIDH VAF data were negative by MRD-NGS at time of best response (16 pts) or subsequently, the latter after a median of 4 (range 2-6) additional cycles of therapy. The median change in mIDH VAF from pre-treatment to time of best response or subsequent MRD-NGS negativity was -19%, corresponding to a median within-pt VAF reduction of 98%. MRD negativity by FC and mIDH NGS corresponded to quality of marrow response (Figure C). Median DOR was 17.0 months for all pts and 24.1 months when HMA+VEN was given frontline. Median OS was 42.2 months and is stratified by 2017 ELN risk in Figure D. Of the 65 pts treated with HMA+VEN, 34 pts received 37 different IDHi-containing regimens as separate lines of therapy. Most (35/37) IDHi regimens (monotherapy 32%, IDHi+HMA 32%, IDHi+HMA+VEN 27%, IDHi+ICT 8%) were given after progression/relapse on antecedent therapy. After a median of 4 cycles (range 1-23), ORR was 54% (CR 24%, CRi 19%, MLFS 8%, PR 3%); the highest CR+CRi rate (67%) was seen with the IDHi + HMA + VEN combination. The median change in mIDH VAF from pre-treatment to best response was -1%, corresponding to a median within-pt VAF reduction of 31%. Comparing within-patients, subsequent treatment with an IDHi-containing regimen after HMA+VEN led to a salvage response rate of 52%; 10/13 responses were CR/CRi, and 8/13 responses bridged the pt to SCT. In pts who instead received HMA+VEN after lack/loss of response to an IDHi regimen, the salvage response rate was higher at 72%; 6/8 responses were CR/CRi, 5/8 responses bridged the pt to SCT.
Conclusions: While not targeted therapy per se, we confirm that the combination of HMA+VEN leads to an impressive CR/CRi rate with longstanding DOR in IDH1/IDH2 mutated AML, particularly in the frontline setting and in NPM1 co-mutated disease. Although accompanying MRD negativity by flow cytometry is frequent, almost half of pts retain an IDH mutation detectable by NGS, suggesting a role for combination therapy with IDH inhibitors. Additionally, we identify excellent outcomes in the relapsed setting, although the presence of a RAS-pathway or TP53 mutation may confer treatment resistance. The high within-patient salvage response rates when switching between HMA+VEN and IDHi-containing regimens warrant further investigation of combination vs. sequential therapy approaches.
Konopleva:Cellectis: Research Funding; Genentech: Consultancy, Research Funding; Stemline Therapeutics: Consultancy, Research Funding; AstraZeneca: Research Funding; Calithera: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Rafael Pharmaceutical: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Eli Lilly: Research Funding; Sanofi: Research Funding; Amgen: Consultancy; AbbVie: Consultancy, Research Funding; Ablynx: Research Funding; Ascentage: Research Funding; Agios: Research Funding; Kisoji: Consultancy; Forty-Seven: Consultancy, Research Funding. Kadia:BMS: Honoraria, Research Funding; Incyte: Research Funding; JAZZ: Honoraria, Research Funding; Novartis: Honoraria; Genentech: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Ascentage: Research Funding; Cyclacel: Research Funding; Pulmotec: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Cellenkos: Research Funding; Astra Zeneca: Research Funding; Celgene: Research Funding. Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Issa:Celegene: Research Funding; Syndax: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Alvarado:Tolero Pharmaceuticals: Research Funding; FibroGen: Research Funding; Astex Pharmaceuticals: Research Funding; Daiichi-Sankyo: Research Funding; BerGenBio ASA: Research Funding; Jazz Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; Sun Pharma: Research Funding. Garcia-Manero:Celgene: Consultancy, Honoraria, Research Funding; Onconova: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Research Funding; H3 Biomedicine: Research Funding; Amphivena Therapeutics: Research Funding; Jazz Pharmaceuticals: Consultancy; Merck: Research Funding; Novartis: Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding. Borthakur:Argenx: Consultancy; PTC Therapeutics: Consultancy; BioLine Rx: Research Funding; Incyte: Research Funding; BMS: Research Funding; Cyclacel: Research Funding; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy; Novartis: Research Funding; PTC Therapeutics: Research Funding; GSK: Research Funding; AstraZeneca: Research Funding; Polaris: Research Funding; Xbiotech USA: Research Funding; Oncoceutics: Research Funding; Curio Science LLC: Consultancy; BioTherix: Consultancy; Jannsen: Research Funding; FTC Therapeutics: Consultancy; Abbvie: Research Funding; BioLine Rx: Consultancy. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding. Kantarjian:Delta Fly: Honoraria; Aptitute Health: Honoraria; BioAscend: Honoraria; Amgen: Honoraria, Research Funding; Janssen: Honoraria; Oxford Biomedical: Honoraria; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sanofi: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Research Funding; Ascentage: Research Funding; BMS: Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Immunogen: Research Funding; Adaptive biotechnologies: Honoraria. DiNardo:ImmuneOnc: Honoraria; Syros: Honoraria; Calithera: Research Funding; Agios: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Takeda: Honoraria; Jazz: Honoraria; MedImmune: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees.
Combination therapy with IDH inhibitors for IDH-mutated AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal